Software for Clinical Operations and Quality Management
Small and early-stage companies look to Agatha to manage their clinical, quality, and regulatory processes. No complicated installations. No complex user training. No big invoice. Just easy to use, cost-effective, and highly efficient applications.
It’s NO MYSTERY why everyday, more than 300 companies use AGATHA to manage and track their clinical studies.
A SharePoint or Dropbox folder is not enough…
to manage your clinical trial documentation. But you also don’t want an expensive, difficult-to implement enterprise solution. With Agatha applications, you do more than manage documents.
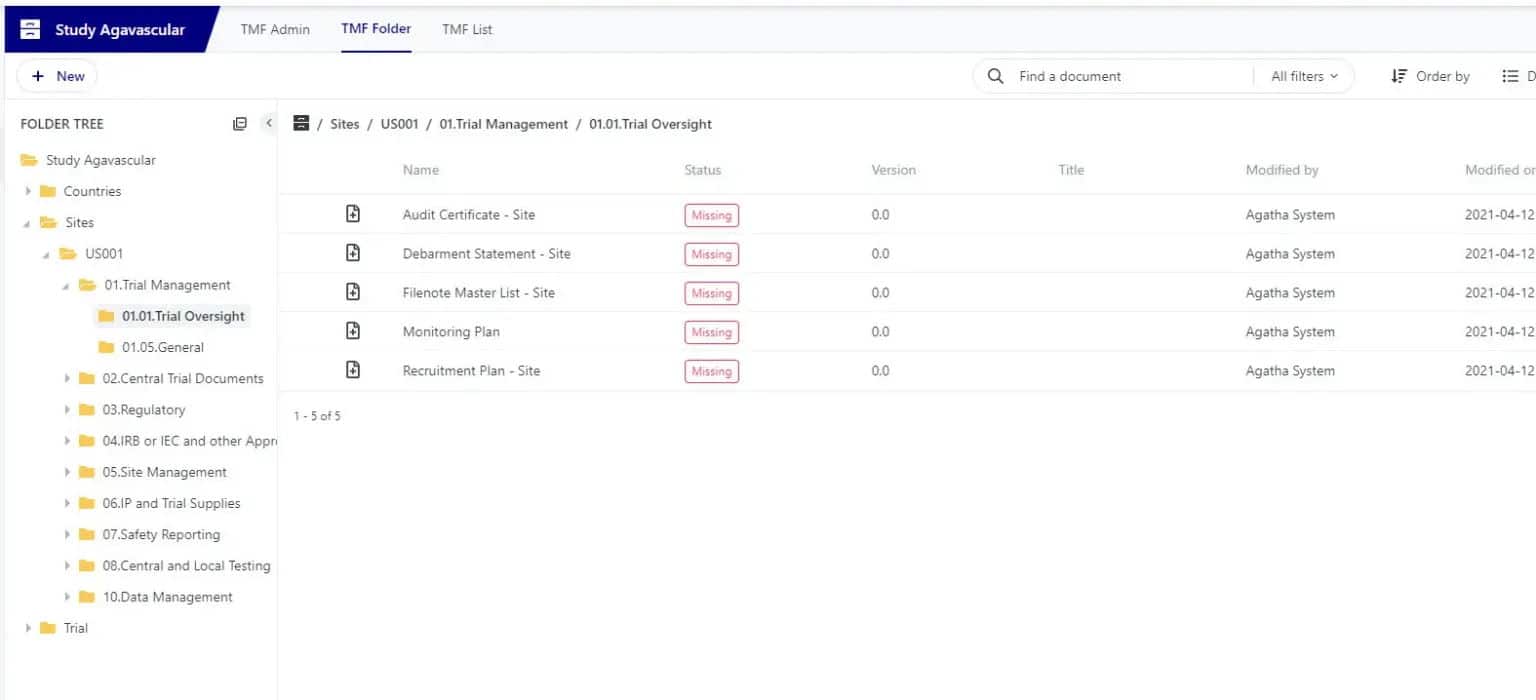
Trial Master File
Cloud management
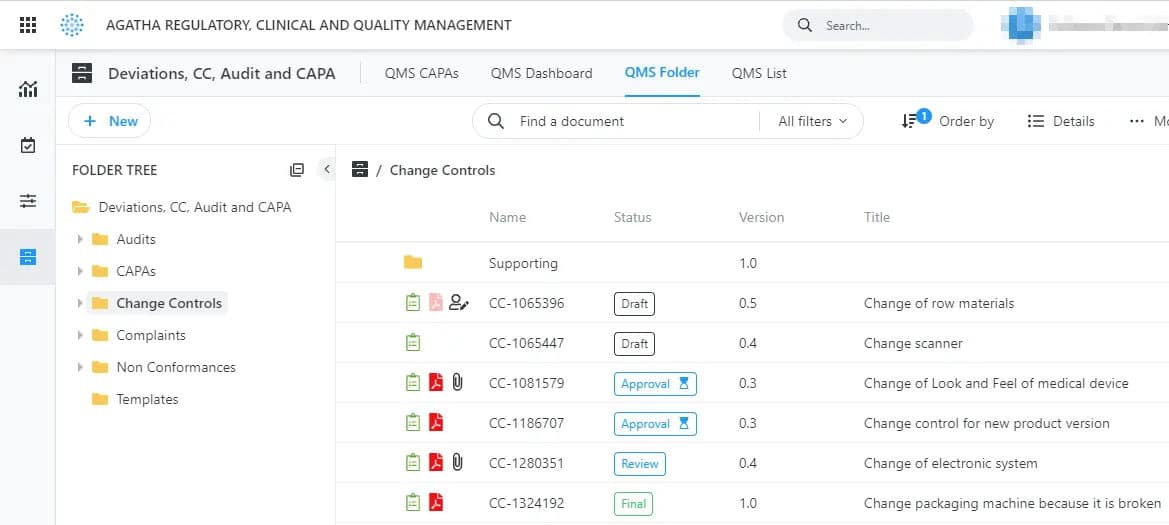
Quality Management
Regulatory Management
Applications for Clinical Operations and Quality Management
Clinical Operations
Quality Management
Medical devices
Our applications for clinical operations
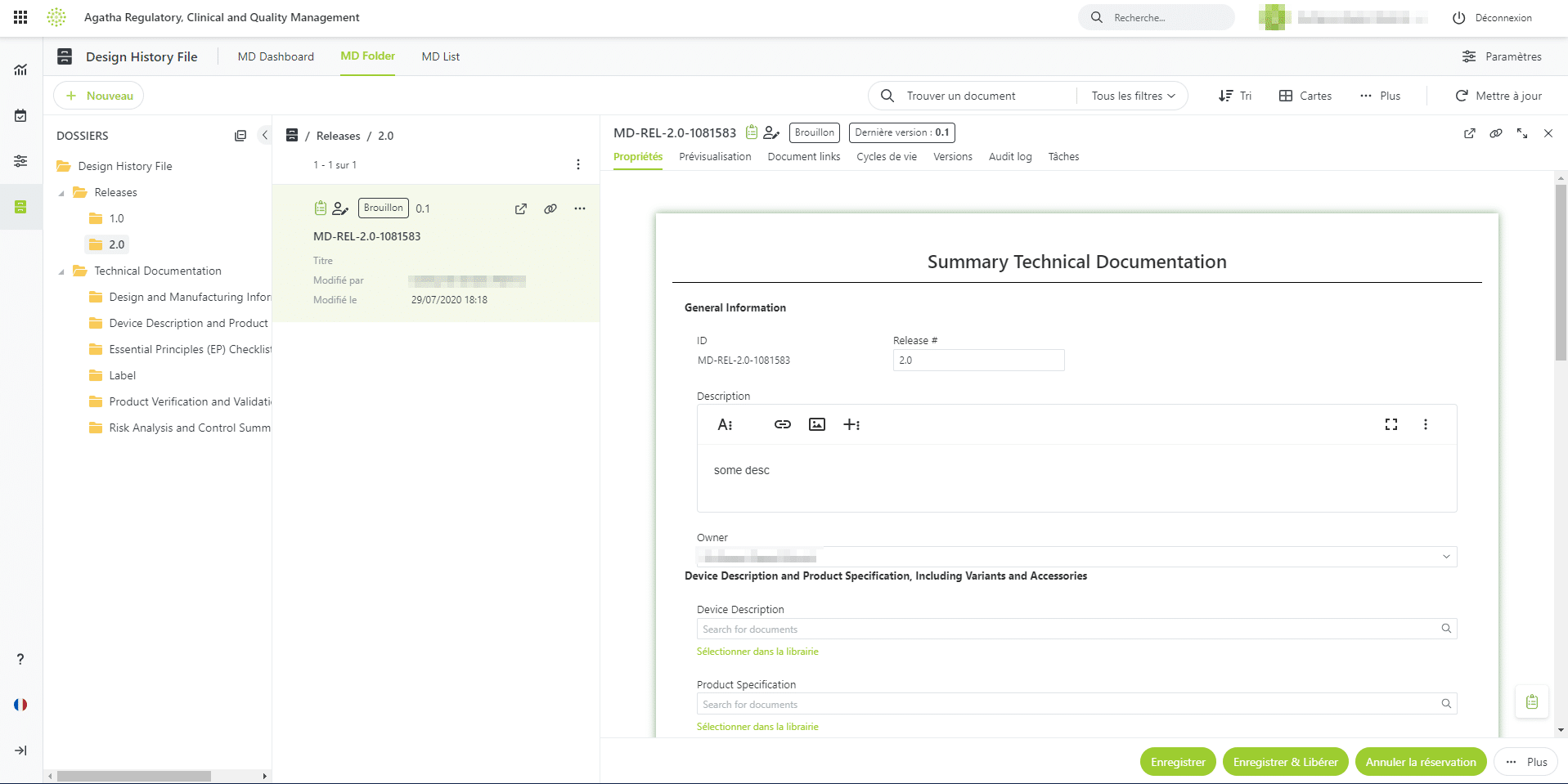
Agatha Clinical eTMF
Ensure every essential document is accounted for with a complete eTMF solution. You can be certain that the set of essential documents you need for every site and study is present and ready for inspection.
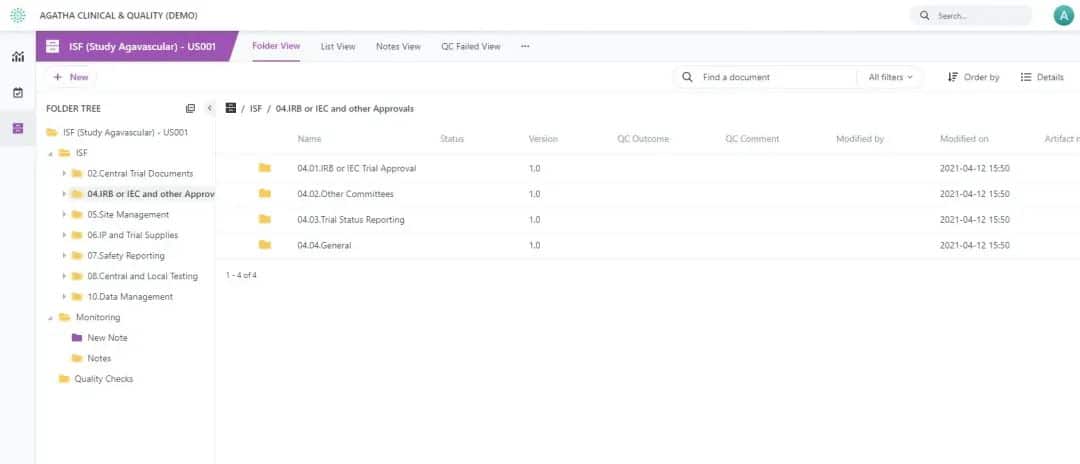
Agatha Remote ISF
Eliminate the need to conduct on-site monitoring visits. You can ensure monitoring activities continue when travel is impossible (like COVID-19) or you want to lower travel costs.
Archita Sharma
Manager des ARCs,
Clinical Research Associate, InCarda Therapeutics
Our applications for quality management
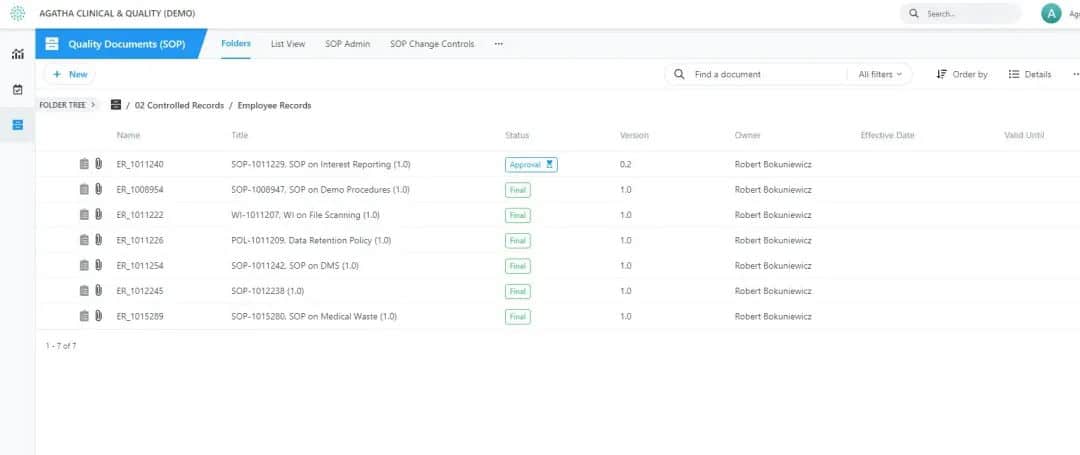
Agatha SOP
Track every activity, collect every signature, and ensure every process is completed. You get a complete set of documents and records, ready for audit at any time.
Agatha Quality Management
Our applications for regulatory management